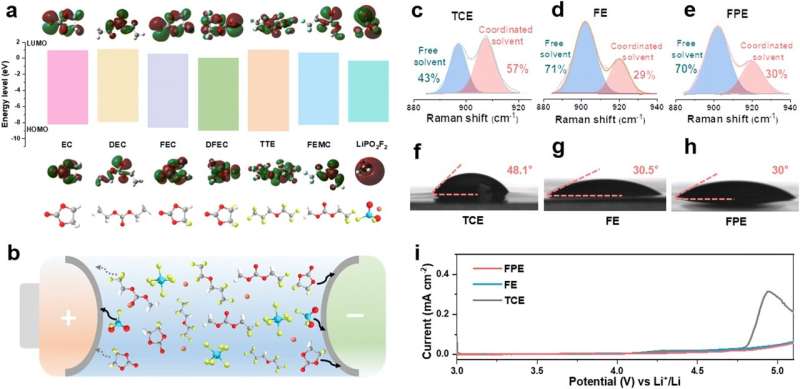
Design precept and properties of fluorinated solvents and purposeful film-forming components in excessive voltage FPE electrolyte. (a) LUMO/HOMO power ranges of various solvents and components in electrolytes. (b) Schematic diagram of the position of every part in stabilizing the interface of the LCO cathode and anode. (c)–(e) Fitted Raman spectra of (c) TCE, (d) FE and (e) FPE. (f)–(h) Contact angles of (f) TCE, (g) FE, and (h) FPE with the separator. (i) LSV curves of Li||LCO cells in these three electrolytes. Credit score: Power & Environmental Science (2024). DOI: 10.1039/D4EE00676C
Lithium-ion batteries (LIBs), primarily used as the ability of pc, communication and client digital merchandise, require larger power density, longer biking life, faster-charging functionality, and a broader working temperature vary to satisfy the rising client calls for.
LiCoO2 (LCO) is the first cathode materials for LIBs. Presently, the superior electrolytes for LCO can not meet the high energy density and fast-charging efficiency of LIBs.
Not too long ago, a analysis group led by Prof. Wu Zhongshuai from the Dalian Institute of Chemical Physics (DICP) of the Chinese language Academy of Sciences (CAS) developed a novel common additive-containing “cocktail electrolyte” primarily based on the synergistic cooperation of multi-component components. This electrolyte enabled business LCO with excessive voltage (4.6 V) and ultra-fast charging (5 C) in a large temperature vary (-20 to 45o C). It additionally exhibited excessive applicability to high-Ni and Co-free cathodes.
The examine was published in Power & Environmental Science.
In precept, rising the charging cutoff voltage can enhance the power density of the batteries. Nevertheless, it will possibly result in the continual oxidative decomposition of electrolytes, extreme progress of non-uniform cathode-electrolyte interphase (CEI), and sluggish interfacial kinetics, which hinders LCO from reaching excessive voltage and quick charging.
To unravel the above issues, the researchers proposed a novel “cocktail electrolyte” (FPE), which may enhance the ultra-stable fast-charging cycle stability of economic LCO at 4.6 V.
They revealed that the cooperation between a number of elements in FPE led to the sturdy and kinetically fats electrode/electrolyte interphases on each cathode and anode. These interfaces, enriched with LiF and Li3AFTER4displayed robust mechanical stability and enhanced ionic conductivity.
Because of this, they prevented cathode floor degradation, suppressed undesirable interfacial reactions, accelerated response kinetics, and mitigated the formation of lithium dendrites even below extraordinarily excessive present densities. Due to this fact, they achieved a high-performance 4.6 V Li-ion battery.
The outcomes confirmed that the capability retention in FPE was as excessive as 73.2%, even at 5 C over 1,000 cycles. In sensible pouch-type cells, this electrolyte enabled graphite||LCO battery to keep up as much as 72.1% capability retention after 2,000 cycles and long-term cyclability over 3,800 cycles.
As well as, the researchers confirmed the final utility of FPE in high-voltage Ni-rich and Co-free cathodes.
“This work provides a practical strategy for high-energy-density and fast-charging batteries,” stated Prof. Wu.
Extra data:
Anping Zhang et al, Regulating electrode/electrolyte interfacial chemistry permits 4.6 V ultra-stable quick charging of economic LiCoO2, Power & Environmental Science (2024). DOI: 10.1039/D4EE00676C
Offered by
Chinese Academy of Sciences
Quotation:
Common ‘cocktail electrolyte’ developed for 4.6 V ultra-stable quick charging of economic lithium-ion batteries (2024, April 18)
retrieved 18 April 2024
from https://techxplore.com/information/2024-04-universal-cocktail-electrolyte-ultra-stable.html
This doc is topic to copyright. Other than any honest dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.