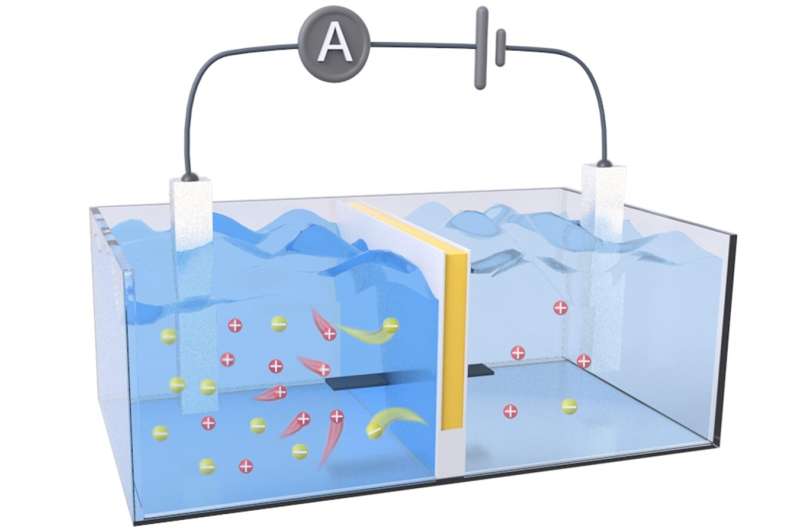
An improved membrane (yellow line) dramatically elevated the quantity of osmotic energy harvested from salt gradients, like these present in estuaries the place salt water (left tank) meets contemporary water (proper tank). Credit score: Tailored from ACS Power Letters 2024, DOI: 10.1021/acsenergylett.4c00320
Estuaries—the place freshwater rivers meet the salty sea—are nice places for birdwatching and kayaking. In these areas, waters containing completely different salt concentrations combine and could also be sources of sustainable, “blue” osmotic vitality.
Researchers in ACS Power Letters report making a semipermeable membrane that harvests osmotic vitality from salt gradients and converts it to electrical energy. The brand new design had an output power density greater than two occasions greater than business membranes in lab demonstrations.
Osmotic vitality might be generated wherever salt gradients are discovered, however the out there applied sciences to seize this renewable energy have room for enchancment. One technique makes use of an array of reverse electrodialysis (RED) membranes that act as a type of “salt battery,” producing electrical energy from strain variations attributable to the salt gradient.
To even out that gradient, positively charged ions from seawater, corresponding to sodium, circulation by way of the system to the freshwater, rising the strain on the membrane. To additional improve its harvesting energy, the membrane additionally must maintain a low inside electrical resistance by permitting electrons to simply circulation in the wrong way of the ions.
Earlier analysis means that enhancing each the circulation of ions throughout the RED membrane and the effectivity of electron transport would seemingly improve the quantity of electrical energy captured from osmotic vitality. So, Dongdong Ye, Xingzhen Qin and colleagues designed a semipermeable membrane from environmentally pleasant supplies that may theoretically reduce inside resistance and maximize output energy.
The researchers’ RED membrane prototype contained separate (i.e., decoupled) channels for ion transport and electron transport. They created this by sandwiching a negatively charged cellulose hydrogel (for ion transport) between layers of an natural, electrically conductive polymer known as polyaniline (for electron transport).
Preliminary assessments confirmed their principle that decoupled transport channels resulted in greater ion conductivity and decrease resistivity in comparison with homogenous membranes created from the identical supplies.
In a water tank that simulated an estuary surroundings, their prototype achieved an output energy density 2.34 occasions greater than a business RED membrane and maintained efficiency throughout 16 days of continuous operation, demonstrating its long-term, steady efficiency underwater. In a ultimate check, the workforce created a salt battery array from 20 of their RED membranes and generated sufficient electrical energy to individually energy a calculator, LED gentle and stopwatch.
Ye, Qin and their team members say their findings increase the vary of ecological supplies that may very well be used to make RED membranes and enhance osmotic energy-harvesting efficiency, making these techniques extra possible for real-world use.
Extra data:
Decoupled Ionic and Digital Pathways for Enhanced Osmotic Power Harvesting, ACS Power Letters (2024). DOI: 10.1021/acsenergylett.4c00320
Offered by
American Chemical Society
Quotation:
Salt battery harvests osmotic vitality the place the river meets the ocean (2024, April 24)
retrieved 28 April 2024
from https://techxplore.com/information/2024-04-salt-battery-harvests-osmotic-energy.html
This doc is topic to copyright. Other than any honest dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.