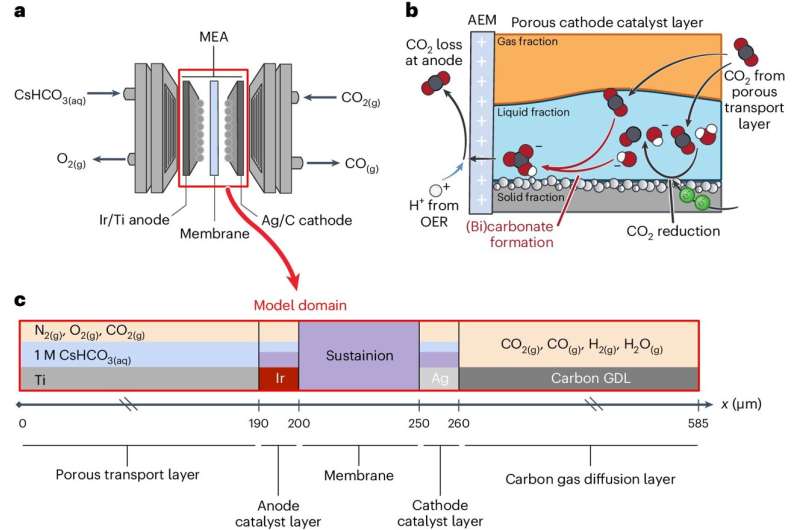
Electrolyzer structure and mannequin area. Credit score: Nature Chemical Engineering (2024). DOI: 10.1038/s44286-024-00062-0
Some elements of the world have been so profitable in making cheap renewable electrical energy that we sometimes have an excessive amount of of it. One attainable use for that low-cost power: Changing carbon dioxide into gasoline and different merchandise utilizing a tool referred to as a membrane-electrode meeting.
A group of scientists from Lawrence Berkeley Nationwide Laboratory (Berkeley Lab) and the College of California Berkeley have developed a brand new strategy to understanding this promising expertise through physics modeling. The paper, which was published within the journal Nature Chemical Engineeringmay assist scientists learn to enhance membrane-electrode meeting effectivity.
Carbon dioxide could be reworked into helpful feedstocks comparable to carbon monoxide and ethylene, which producers use to make merchandise together with chemical compounds and packaging. A method to do that is with membrane-electrode assemblies, that are gadgets that include two electrodes separated by a membrane.
Additionally utilized in gasoline cells that flip inputs like hydrogen into electrical energy, membrane-electrode assemblies maintain promise for having the ability to use surplus renewable energy to run response sequences that catalyze carbon dioxide into different chemical compounds. However these gadgets have issues with effectivity, and their workings should not but totally understood.
“Membrane-electrode assemblies are complicated systems with multiple layers. Each layer holds different chemical species, additives, and particles,” stated Adam Weber, a senior scientist at Berkeley Lab and corresponding writer of the research.
“Often, we don’t really know why experiments with membrane-electrode assemblies produce certain products, or why they fail to convert a larger percentage of a given amount of carbon dioxide.”
Berkeley Lab scientists have developed a digital mannequin to speed up the optimization of membrane-electrode assemblies to transform CO2 to gasoline and different merchandise. Credit score: Justin Bui, Francisco Galang and Samantha Trieu/Berkeley Lab
Laptop modeling may also help predict which system parameters will produce the very best outcomes, however they are usually much less correct at anticipating points comparable to crossover, which is when carbon dioxide strikes throughout the membrane as a substitute of reacting. To enhance mannequin accuracy, the researchers turned to Marcus–Hush–Chidsey kinetics, a concept that beforehand had not been built-in into membrane-electrode meeting modeling and is proven to be vital for understanding the response mechanism.
The researchers validated their mannequin towards experimental datadiscovering that it did a greater job of predicting real-world outcomes than earlier fashions. Amongst different benefits, the usage of Marcus–Hush–Chidsey kinetics made it attainable to account for the function of water orientation.
The group then ran digital experiments with its mannequin to discover how totally different membrane-electrode meeting designs carried out when it comes to carbon-dioxide utilization and selectivity for desired merchandise. “With this work, we have shown how you can leverage chemical engineering principles toward these advanced technologies that are coming online,” he stated. “That gives us insights and ideas for optimizing these cell designs and materials so we can go forward and make them.”
Among the variables the group examined just about included catalyst-layer thickness and catalyst-specific floor space. In addition they uncovered design guidelines across the significance of coupled ion and water transportin addition to tradeoffs between transport phenomena and response and buffer kinetics. All of those change the general power effectivity, merchandise obtained, and quantity of carbon dioxide transformed.
“Having a digital twin of a system allows you to probe a much larger parameter space much more rapidly than in experiments, which are typically complex and require special equipment,” Weber stated, including, “We can’t see where every molecule is in an experiment. But in a model, we can.”
Weber stated the following step within the analysis is to extend the mannequin’s complexity to have the ability to have a look at efficiency over a membrane-electrode meeting’s lifetime, amongst different variables.
Extra data:
Eric W. Lees et al, Exploring CO2 discount and crossover in membrane electrode assemblies, Nature Chemical Engineering (2024). DOI: 10.1038/s44286-024-00062-0
Supplied by
Lawrence Berkeley National Laboratory
Quotation:
A greater mannequin for changing carbon dioxide into fuels and merchandise (2024, June 3)
retrieved 3 June 2024
from https://techxplore.com/information/2024-06-carbon-dioxide-fuels-products.html
This doc is topic to copyright. Aside from any honest dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.