Join daily news updates from CleanTechnica on e-mail. Or follow us on Google News!
Manganese is earth-abundant and low-cost. A brand new course of may assist make it a contender to exchange nickel and cobalt in batteries.
Rechargeable lithium-ion batteries are rising in adoption, utilized in units like smartphones and laptops, electrical automobiles, and power storage programs. However provides of nickel and cobalt generally used within the cathodes of those batteries are restricted. New analysis led by the Division of Vitality’s Lawrence Berkeley Nationwide Laboratory (Berkeley Lab) opens up a possible low-cost, secure various in manganese, the fifth most plentiful metallic within the Earth’s crust.
Researchers confirmed that manganese might be successfully utilized in rising cathode supplies referred to as disordered rock salts, or DRX. Earlier analysis steered that to carry out nicely, DRX supplies needed to be floor all the way down to nanosized particles in an energy-intensive course of. However the brand new research discovered that manganese-based cathodes can truly excel with particles which can be about 1000 instances bigger than anticipated. The work was printed Sept. 19 within the journal Nature Nanotechnology.
“There are many ways to generate power with renewable energy, but the importance lies in how you store it,” stated Han-Ming Hau, who researches battery expertise as a part of Berkeley Lab’s Ceder Group and is a PhD scholar at UC Berkeley. “By applying our new approach, we can use a material that is both earth-abundant and low-cost, and that takes less energy and time to produce than some commercialized Li-ion battery cathode materials. And it can store as much energy and work just as well.”
The researchers used a novel two-day course of that first removes lithium ions from the cathode materials after which heats it at low temperatures (about 200 levels Celsius). This contrasts with the present course of for manganese-based DRX supplies, which takes greater than three weeks of remedy.
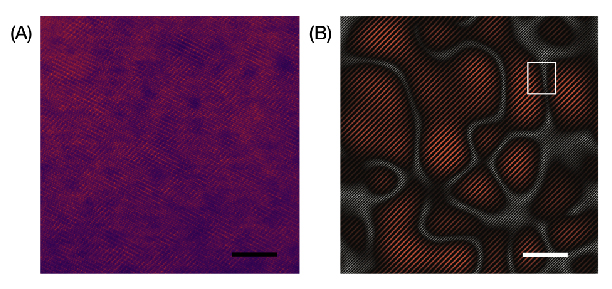
Researchers used state-of-the-art electron microscopes to seize atomic-scale footage of the manganese-based materials in motion. They discovered that after making use of their course of, the fabric shaped a nanoscale semi-ordered construction that really enhanced the battery efficiency, permitting it to densely retailer and ship power.
The group additionally used totally different methods with X-rays to review how battery biking causes chemical adjustments to manganese and oxygen on the macroscopic degree. By learning how the manganese materials behaves at totally different scales, the group opens up totally different strategies for making manganese-based cathodes and insights into nano-engineering future battery supplies.
“We now have a better understanding of the unique nanostructure of the material,” Hau stated, “and a synthesis process to cause this ‘phase change’ in the material that improves its electrochemical performance. It’s an important step that pushes this material closer to battery applications in the real world.”
This analysis used assets at three DOE Workplace of Science person services: the Advanced Light Source and Molecular Foundry (National Center for Electron Microscopy) at Berkeley Lab, and the National Synchrotron Light Source II at Brookhaven Nationwide Laboratory. The work was supported by DOE’s Workplace of Vitality Effectivity and Renewable Vitality and Workplace of Science.
Courtesy of Lawrence Berkeley National Laboratory (Berkeley Lab). By Lauren Biron.
Have a tip for CleanTechnica? Wish to promote? Wish to counsel a visitor for our CleanTech Speak podcast? Contact us here.
Newest CleanTechnica.TV Movies
CleanTechnica makes use of affiliate hyperlinks. See our coverage here.
CleanTechnica’s Comment Policy