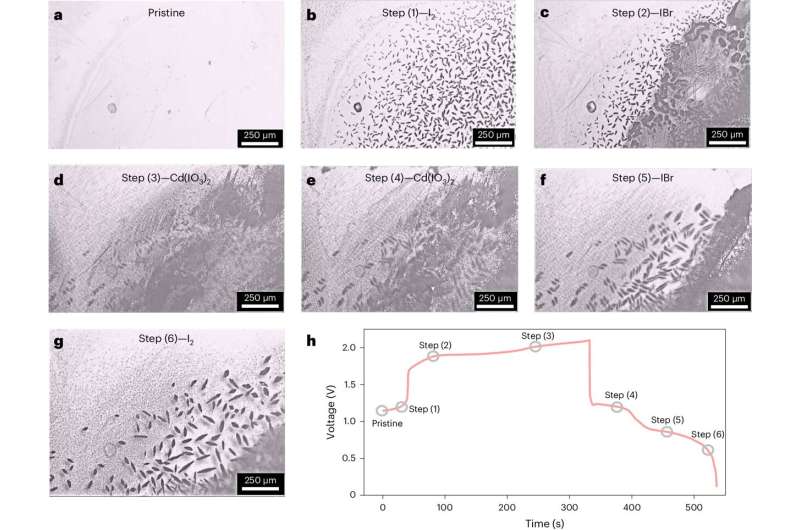
In situ remark of the charge-discharge strategy of IBA underneath an optical microscope. Credit score: Nature Power (2024). DOI: 10.1038/s41560-024-01515-9
Conventional non-aqueous lithium-ion batteries have a excessive vitality density, however their security is compromised because of the flammable natural electrolytes they make the most of.
Aqueous batteries use water because the solvent for electrolytes, considerably enhancing the protection of the batteries. Nevertheless, because of the restricted solubility of the electrolyte and low battery voltage, aqueous batteries sometimes have a decrease vitality density. Which means that the quantity of electrical energy saved per unit quantity of aqueous battery is comparatively low.
In a examine printed in Nature Energya analysis group led by Prof. Li Xianfeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese language Academy of Sciences (CAS), in collaboration with Prof. Fu Qiang’s group additionally from DICP, developed a multi-electron switch cathode based mostly on bromine and iodinerealizing a selected capability of greater than 840 Ah/L, attaining an vitality density of as much as 1,200 Wh/L based mostly on catholyte in full battery testing.
To enhance the vitality density of aqueous batteries, researchers used a blended halogen answer of iodide ions (I–) and bromide ions (Br–) because the electrolyte. They developed a multi-electron switch response, transferring I– to iodine aspect (I2) after which to iodate (IO3–).
Throughout the charging course of, I– had been oxidized to IO3– on the constructive facet, and the generated H+ had been performed to the destructive facet within the type of supporting electrolyte. Throughout the discharge course of, H+ had been performed from the constructive facet, and IO3– had been lowered to I–.
The developed multi-electron switch cathode had a selected capability of 840 Ah/L. Combining the cathode with metallic Cd to kind a full battery, researchers achieved an vitality density as much as 1,200 Wh/L based mostly on the developed catholyte.
Researchers confirmed that Br– added to the electrolyte might generate polar iodine bromide (IBr) through the charging course of, which facilitated the response with H2O to kind IO3–. Throughout the discharge, IO3– might oxidize Br– to Br2 and took part within the electrochemical response to appreciate reversible and speedy discharge of IO3–.
Due to this fact, the bromide intermediate fashioned through the cost and discharge course of optimized the response course of, successfully enhancing the kinetic and reversibility of the electrochemical response.
Prof. Fu’s group proved the multi-electron switch course of by in-situ optical microscopyRaman spectroscopy and so forth.
“This examine gives a brand new thought for the design of high-energy-density aqueous batteries, and will broaden the aqueous batteries utility in energy batteries discipline,” stated Prof. Li.
Extra info:
Xie, C., et al. Reversible multielectron switch I−/IO3− cathode enabled by a hetero-halogen electrolyte for high-energy-density aqueous batteries. Nature Power (2024). DOI: 10.1038/s41560-024-01515-9
Supplied by
Chinese Academy of Sciences
Quotation:
Researchers develop high-energy-density aqueous battery based mostly on halogen multi-electron switch (2024, April 23)
retrieved 24 April 2024
from https://techxplore.com/information/2024-04-high-energy-density-aqueous-battery.html
This doc is topic to copyright. Other than any truthful dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is offered for info functions solely.