College of Toronto Engineering Ph.D. college students Rui Kai (Ray) Miao (left) and Panos Papangelakis (proper) maintain up a brand new catalyst they designed to transform captured CO2 gasoline into precious merchandise. Their model performs effectively even within the presence of sulfur dioxide, a contaminant that toxins different catalysts. Credit score: Tyler Irving / College of Toronto Engineering
A newly designed catalyst created by College of Toronto Engineering researchers effectively converts captured carbon into precious merchandise—even within the presence of a contaminant that degrades the efficiency of present variations.
The invention is a vital step towards extra economically favorable methods for carbon seize and storage that could possibly be added on to current industrial processes.
“Today, we have more and better options for low-carbon electricity generation than ever before,” says Professor David Sinton, senior writer on a paper printed in Nature Vitality that describes the brand new catalyst.
“But there are other sectors of the economy that will be harder to decarbonize: for example, steel and cement manufacturing. To help those industries, we need to invent cost-effective ways to capture and upgrade the carbon in their waste streams.”
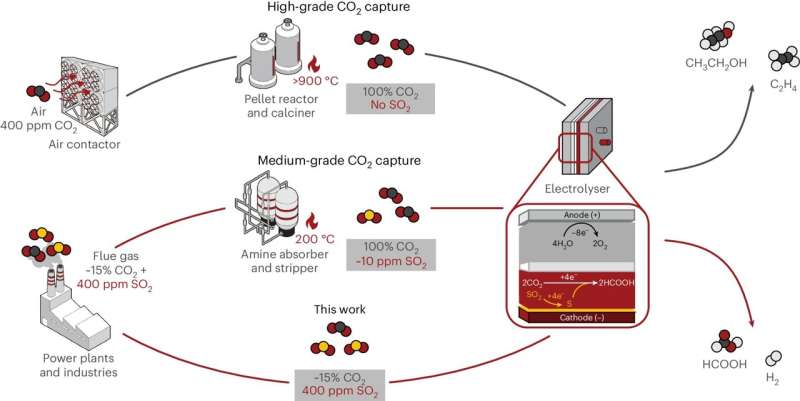
Sinton and his staff use units generally known as electrolyzers to transform CO2 and electrical energy into merchandise equivalent to ethylene and ethanol. These carbon-based molecules will be bought as fuels or used as chemical feedstocks for making on a regular basis objects equivalent to plastic.
Contained in the electrolyzer, the conversion response occurs when three parts—CO2 gasoline, electrons and a water-based liquid electrolyte—come collectively on the floor of a stable catalyst.
The catalyst is usually manufactured from copper however might also comprise different metals or natural compounds that may additional enhance the system. Its operate is to hurry up the response and decrease the creation of undesirable aspect merchandise, equivalent to hydrogen gasoline, which scale back the effectivity of the general course of.
Whereas many groups world wide have produced high-performing catalysts, practically all of them are designed to function with a pure CO2 feed. But when the carbon in query comes from smokestacks, the feed is more likely to be something however pure.
“Catalyst designers generally don’t like dealing with impurities, and for good reason,” says Panos Papangelakis, a Ph.D. pupil in mechanical engineering and one in all 5 co-lead authors on the brand new paper.
“Sulfur oxides, equivalent to SO2poison the catalyst by binding to the floor. This leaves fewer websites for CO2 to react, and it additionally causes the formation of chemical compounds you don’t need.
“It happens really fast: whereas some catalysts can last hundreds of hours on a pure feed, if you introduce these impurities, within minutes they can be down to 5% efficiency.”
Processing of CO2 gasoline. The seize and electrolysis of CO2 from air or flue gasoline streams and the impact of SO2 poisoning on the response. e–electron. Credit score: Nature Vitality (2024). DOI: 10.1038/s41560-024-01577-9
Although there are well-established strategies to take away impurities from CO2-rich exhaust gases earlier than feeding them into the electrolyzer, they take time, require vitality and lift the price of carbon capture and upgrading. Moreover, within the case of SO2even a bit of bit generally is a large downside.
“Even if you bring your exhaust gas down to less than 10 parts per million, or 0.001% of the feed, the catalyst can still be poisoned in under 2 hours,” says Papangelakis.
Within the paper, the staff describes how they went about designing a extra resilient catalyst that would stand as much as SO2 by making two key modifications to a typical copper-based catalyst.
On one aspect, they added a skinny layer of polyteterafluoroethylene, often known as Teflon. This non-stick materials modifications the chemistry on the catalyst floor, impeding the reactions that allow SO2 poisoning to happen.
On the opposite aspect, they added a layer of Nafion, an electrically-conductive polymer typically utilized in gasoline cells. This advanced, porous materials incorporates some areas which can be hydrophilic, which means they entice water, in addition to different areas which can be hydrophobic, which means they repel water. This construction makes it tough for SO2 to succeed in the catalyst floor.
The staff then fed this catalyst with a mixture of CO2 and SO2with the latter at a focus of about 400 components per million, typical of an industrial waste stream. Even beneath these robust circumstances, the brand new catalyst carried out effectively.
“In the paper, we report a Faraday efficiency—a measure of how many of the electrons ended up in the desired products—of 50%, which we were able to maintain for 150 hours,” says Papangelakis.
“There are some catalysts out there that might start at a higher efficiency, maybe 75% or 80%. But again, if you expose them to SO2within minutes or at most a couple of hours, that drops down to almost nothing. We were able to resist that.”
Papangelakis says that as a result of his staff’s strategy does not have an effect on the composition of the catalyst itself, it needs to be extensively relevant. In different phrases, groups which have already perfected high-performing catalysts ought to be capable to use related coatings to confer resistance to sulfur oxide poisoning.
Though sulfur oxides are essentially the most difficult impurity in typical waste streams, they don’t seem to be the one ones, and it is the complete set of chemical contaminants that the staff is popping to subsequent.
“There are lots of other impurities to consider, such as nitrogen oxides, oxygen, etc.,” says Papangelakis.
“But the fact that this approach works so well for sulfur oxides is very promising. Before this work, it was just taken for granted that you’d have to remove the impurities before upgrading CO2. What we’ve shown is that there might be a different way to deal with them, which opens up a lot of new possibilities.”
Extra data:
Panagiotis Papangelakis et al, Enhancing the SO2 tolerance of CO2 discount electrocatalysts utilizing a polymer/catalyst/ionomer heterojunction design, Nature Vitality (2024). DOI: 10.1038/s41560-024-01577-9
Supplied by
University of Toronto
Quotation:
New contaminant-tolerant catalyst may assist seize carbon immediately from smokestacks (2024, July 5)
retrieved 5 July 2024
from https://techxplore.com/information/2024-07-contaminant-tolerant-catalyst-capture-carbon.html
This doc is topic to copyright. Aside from any truthful dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for data functions solely.
