Graphical summary. Credit score: Vitality & Fuels (2024). DOI: 10.1021/acs.energyfuels.4c01232
Industrial emissions are one of many important sources of local weather change-inducing carbon dioxide (CO2). Whereas adopting renewable and clear power alternate options is one choice for mitigating these carbon emissions, carbon seize expertise is one other resolution to manage CO2 emissions.
In massive CO2-emitting industries, akin to cement, oil refineriesand thermal energy crops, carbon capture technology could be simply utilized to take away CO2 emissions instantly on the supply at a possible value and with low power consumption. Totally different supplies have been explored for CO2 seize in factories, together with zeolites, metallic−natural frameworks, pure minerals, alkalis, and alkali metallic salts. Amongst them, alkali metallic carbonates, akin to sodium carbonate (That2CO3), are thought of efficient and cheap supplies with secure properties and straightforward procurement.
Theoretically, Na2CO3 has a good CO2 seize capability and could be simply regenerated for successive makes use of. Nevertheless, instantly making use of Na2CO3 to seize CO2 causes crystal agglomeration, resulting in poor effectivity and shorter longevity. This challenge could be eradicated through the use of a carbon skeleton for Na2CO3.
Porous carbon supplies with good pore connectivity present low density, structural stabilityhydrophobicity, and a big floor space that may stabilize Na2CO3. Earlier research report that Na2CO3−carbon nanocomposites have a CO2 seize capability of 5.2 mmol/g. Nevertheless, these research don’t examine the impact of the carbonization temperatures on the general efficiency of the fabric.
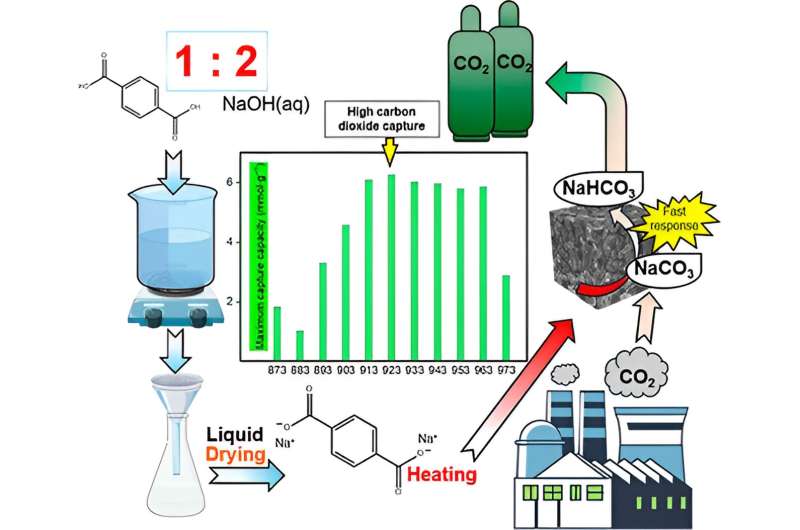
In a examine printed in Energy & Fuels on June 12, 2024, Professor Hirofumi Kanoh and Bo Zhang from the Graduate College of Science, Chiba College, synthesized a hybrid CO2 seize materials consisting of Na2CO3 wrapped with porous nanocarbon.
They additional evaluated its CO2 seize and regeneration efficiencies at completely different carbonization temperatures. The Na2CO3−carbon hybrids (NaCH) have been derived by carbonization of disodium terephthalate at temperatures starting from 873K to 973 Ok within the presence of nitrogen as a protecting fuel.
“Reducing CO2 emissions is an urgent issue, but research on the methods and material systems for CO2 capture are still lacking. This Na2CO3−carbon hybrid system proved promising in our initial investigations, prompting us to explore it further,” states Prof. Kanoh.
The crew measured the hybrid supplies’ CO2 seize capability below humid conditions to imitate the circumstances of manufacturing facility waste exhaust gases. They discovered that the NaCH hybrids ready at carbonization temperatures close to 913–943 Ok demonstrated larger CO2 seize capacities.
Amongst them, NaCH-923 had the best CO2 seize capability of 6.25 mmol/g and a excessive carbon content material of over 40%, which resulted in a bigger floor space, enabling a extra uniform distribution of Na2CO3 on the nanocarbon floor. This decreased the speed of Na2CO3 crystal agglomeration and led to sooner response charges.
After NaCH-923 successfully captured CO2the scientists once more heated the resultant NaCH-923-CO2 within the presence of nitrogen to check its regeneration efficiency. They discovered that NaCH-923 may very well be regenerated and used for CO2 seize for 10 cycles, whereas retaining over 95% of its preliminary CO2 seize capability. These outcomes point out that NaCH-923 reveals good structural power, sturdiness, and regeneration, which makes it a wonderful materials for CO2 seize below humid circumstances.
Additional experiments on the NaCH-923-CO2 confirmed that the pattern underwent a steep mass change at 326−373 Ok (round 80 °C on common). Because the temperature of the exhaust fuel from thermal energy crops can also be sometimes in that vary, the waste warmth from factories and energy crops can simply be used as a heat source for regenerating NaCH-923, thereby successfully lowering power consumption.
These findings present that the carbonization temperature considerably influences the CO2 seize efficiency and carbon content material of NaCH hybrids, with NaCH-923 exhibiting the very best traits. NaCH-923, being a strong adsorbent, can effectively seize CO2 at ambient temperature and strain with excessive selectivity for CO2 and with out the issue of apparatus corrosion that exists with liquid adsorbents at present utilized in industries.
Furthermore, these traits enable for its widespread software in varied configurations, environments, and numerous industrial settings.
“By transforming Na2CO3which already has a good CO2 capture capacity, into a nanocomposite, it became possible to improve the reaction rate and reduce the decomposition and regeneration temperature. This enables the use of factory waste heat for regeneration at around 80 °C, giving us an energy-cost efficient CO2 capture system,” concludes Prof. Kanoh.
Extra data:
Bo Zhang et al, Sodium Carbonate–Carbon Hybrid Materials for Low-Vitality-Consuming CO2 Seize, Vitality & Fuels (2024). DOI: 10.1021/acs.energyfuels.4c01232
Supplied by
Chiba University
Quotation:
Capturing carbon with energy-efficient sodium carbonate−nanocarbon hybrid materials (2024, July 16)
retrieved 16 July 2024
from https://techxplore.com/information/2024-07-capturing-carbon-energy-efficient-sodium.html
This doc is topic to copyright. Aside from any truthful dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for data functions solely.